Welcome to the
WODIA PROJECT
Personalized Medicine Screening and Monitoring Programme for Pregnant Women Suffering from Preeclampsia and Gestational Hypertension
Learn more about the WODIA research project
Contact us Discover WODIA
Learn more about the WODIA research project
Contact us Discover WODIA
With the WODIA project we aim to make it safer for women to give birth by identifying and tracking early signs of preeclampsia while it is still preventable with a personalized medicine screening, therapy, and home monitoring service.
The service will combine the maternal phenotype with the measured biomarkers. This will allow for more effective targeted personalized medicine with individual medication dosing and fewer and more effective clinical visits. We will do this by developing and evaluating a fully automated information and communication technology-based screening and home monitoring service with a machine learning based decision support system as backend.
Maternal phenotype and characteristics will be combined with biomarkers measured in the home and clinical setting to adjust medication continuously.
We will evaluate WODIA in a clinical feasibility trial.
Contact us Background
Half a million women die each year giving birth. Preeclampsia and gestational hypertension alone are the cause of 76,000 mothers and ½ million infants dying each year. Infants who survive often experience long-term health problems, including cerebral palsy, chronic lung disease, blindness and hearing loss, and the resulting societal healthcare costs are high.
Preeclampsia complicates up to 8% of all pregnancies worldwide and can lead to several life-threatening conditions, often requiring medical treatment and hospitalization. Risks for the fetus include poor growth and preterm birth. The cost of preeclampsia is estimated to be up to 13.5 billion a year in the European Union alone.
Once preeclampsia is diagnosed it cannot be cured. Recent studies have shown promising new diagnostic approaches using an extensive screening and monitoring battery of biomarkers combined with maternal phenotype and history categorization which are then classified into a risk model. Based on this model, medical treatment with antiplatelet agents have been found to be effective in a cohort of more than 61,000 pregnancies.
Contact us MethodsThe WODIA project features both technical research and development activities combined with a clinical feasibility trial for validating the technology and providing insights into practicality and feasibility of the project.
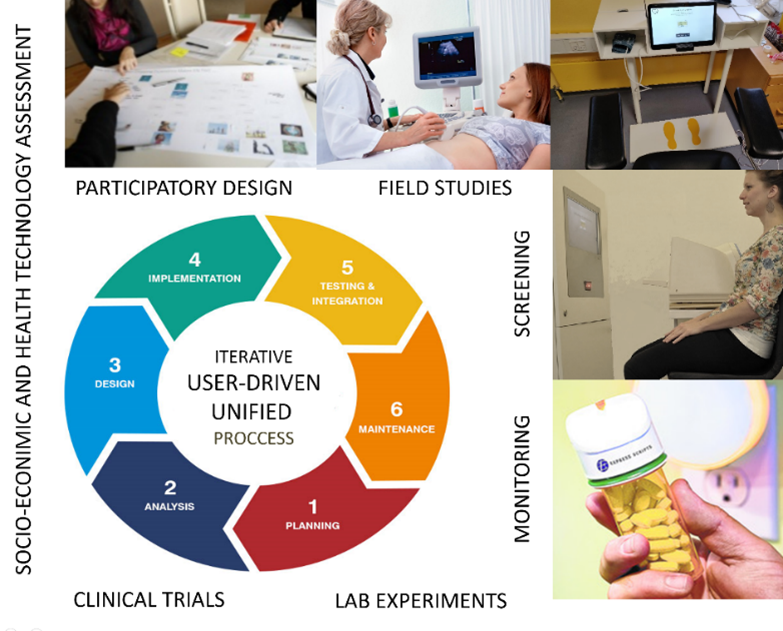
WODIA research approach and methodology relies on user-driven methods, including participatory design and co-creation using mixed methods with field studies and interviews, combined with prototyping and software development. All handled within an iterative unified process (UP) methodology to ensure timeliness and quality of all project tasks.
The use of machine learning and articifical intelligence (AI) is a major tool for the project, as it will allow us to detect the patterns of biomarkers and maternal background that leads to preeclampsia and gestational hypertension.
In order to validate WODIA’s tools and services, evidence-based medicine methods are employed as part of a multicenter study where we plan to include 1.000 pregnant women in a clinical feasibility trial in Denmark and Poland.
The clinical trial will help us refine the AI algorithms further, and will provide us with both qualitative and qualitative insights into the user experience of patients, relatives, and staff, and create more user freindly and understandable system.
WODIA will recruit pregnant women attending the pregancy clinics at Aarhus University Hospital or Gameta. Participation is complety voluntary (see ethics section) and the inclusion and exclusion criteria are highlighted below.
Inclusion criteria:
[1] Duley, L., The global impact of pre-eclampsia and eclampsia. Seminars in perinatology, 2009. 33(3): p. 130-137. DOI: 10.1053/j.semperi.2009.02.010 [2] International, W.H.O., Geographic variation in the incidence of hypertension in pregnancy. World Health Organization International Collaborative Study of Hypertensive Disorders of Pregnancy. American Journal of Obstetrics and Gynecology, 1988. 158(1): p. 80-83. [3] Harmon QE, Huang L, Umbach DM, et al. Risk of fetal death with preeclampsia. Obstet Gynecol 2015; 125:628. [4] Rolnik, D.L. , Wright D., Poon, L.C.Y. Syngelaki A., O'Gorman, N , de Paco Matallana, C., Akolekar C., Cicero, S. , Janga, D. , Singh, M., Molina, F.S. , Persico, N., Jani, J.C., Plasencia W , Papaioannou, G , Tenenbaum-Gavish, K. , Nicolaides, K.H. ASPRE Trial: Performance of Screening for Preterm Pre-Eclampsia Ultrasound Obstet Gynecol. 2017 Oct;50(4):492-495. doi: 10.1002/uog.18816. Epub 2017 Aug 24. [5] Tan MY, Wright D, Syngelaki A, Akolekar R, Cicero S, Janga D, Singh M, Greco E, Wright A, Maclagan K, Poon LC, Nicolaides KH. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstet Gynecol. 2018 Jun;51(6):743-750. doi: 10.1002/uog.19039. [6] LJ, Petrou S, Doyle LW, Draper ES, Marlow N. The cost of preterm birth throughout childhood in England and Wales. Mangham Pediatrics. 2009 Feb;123(2):e312-27. doi: 10.1542/peds.2008-1827. https://www.ncbi.nlm.nih.gov/pubmed/19171583 [7] Carty, D. M., Delles, C., & Dominiczak, A. F. (2008). Novel biomarkers for predicting preeclampsia. Trends in cardiovascular medicine, 18(5), 186-194. [8] Steinthorsdottir, V., McGinnis, R., Williams, N.O. et al. Genetic predisposition to hypertension is associated with preeclampsia in European and Central Asian women. Nat Commun 11, 5976 (2020). https://doi.org/10.1038/s41467-020-19733-6 [9] Roberts, J. M., Rich-Edwards, J. W., McElrath, T. F., Garmire, L., Myatt, L., & Global Pregnancy Collaboration. (2021). Subtypes of Preeclampsia: Recognition and Determining Clinical Usefulness. Hypertension, 77(5), 1430-1441. [10] Llinos Roberts, Piya Chaemsaithong, Daljit S. Sahota, Kypros H. Nicolaides, and Liona C.y. Poon. Protocol for measurement of mean arterial pressure at 10–40 weeks’ gestation. Pregnancy Hypertension, 10:155–160, 2017. doi: 10.1016/j.preghy.2017.08.002 [11] Midori Sasaki Yatabe, Junichi Yatabe, Kei Asayama, Jan A. Staessen, Blerim Mujaj, Lutgarde Thijs, Kyotaro Ito, Tomohiro Sonoo, Satoshi Morimoto, Atsuhiro Ichihara, and et al. The rationale and design of reduction of uncontrolled hypertension by remote monitoring and telemedicine (remote) study. Blood Pressure, 27(2):99–105, 2017. doi: 10.1080/08037051.2017.1406306. [12] Edgardo Abalos, Lelia Duley, D Wilhelm Steyn, and Celina Gialdini. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database of Systematic Reviews, 2018. doi: 10.1002/14651858.cd002252.pub4 [13] Myers JE, Myatt L, Roberts JM, Redman C (2018). COLLECT, a collaborative database for pregnancy and placental research studies worldwide. BJOG. doi: 10.1111/1471-0528.15393.. [14] PRESIDE PROJECT WEB PAGE https://www.rigshospitalet.dk/afdelinger-og-klinikker/julianemarie/obstetrisk-afdeling/forskning/aktuelle-projekter/Sider/preside.aspx. Accessed June 2021 [15] Wagner, S. (2016). Blood pressure self-measurement. Hypertension: from basic research to clinical practice, Springer Link 97-107. [16] Harris, P. A., Taylor, R., Thielke, R., Payne, J., Gonzalez, N., & Conde, J. G. (2009). Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics, 42(2). [17] Jhee JH, Lee S, Park Y, Lee SE, Kim YA, Kang S-W, et al. (2019) Prediction model development of late-onset preeclampsia using machine learning-based methods. PLoS ONE 14 (8): e0221202. [18] Maric I, Tsur A, Aghaeepour N, et al. Early prediction of preeclampsia via machine learning. Am J Obstet Gynecol MFM 2020;2:1001
The study will be performed in accordance with the applicable regulatory requirements/legislation. Permission to perform the study will be sought from the respective Scientific Ethical Committee of both countries, and the study will be reported to the relevant data protection agencies. Participants will be able to withdraw consent at any time.
Contact us PartnersThe following are the partners of WODIA:
Partner AU: is the coordinator specializing in biomedical engineering. They will lead the implementation of the WODIA technical platform including the point-of-care and telemonitoring services. AU has a very close cooperation with partner AUH in terms of performing clinical research.
Partner AUH: is the principal clinical investigator and will lead the clinical feasibility trial, including clearing ethical and data management issues, and coordinating with GAM & the other partners.
Partner GUT: is a technical research partner also specialzinig in biomedical engineering. GUT will mainly be responsible for developing the AI-models based on the data obtained from AUH&GAM.
Partner GAM: is a clinical partner specializing in ultrasound examinations and consultations with pregant women. GAM will participate in the clinical trial in close cooperation with AUH.
Partner ZITEC: is a private industry vendor working in web and smart phone design, including within the telehealth area. ZITEC will develop the user interfaces needed for WODIA:
The project is funded by ERA PerMed and the three partners countries Denmark, Poland and Romania.
ERA PerMed is a ERA-Net Cofund, supported by 32 partners from 23 countries and cofunded by the European Commission.
To align national research strategies, promote excellence, reinforce the competitiveness of European players in PM, and enhance the European collaboration with non-EU countries, national funding organisations have agreed to launch Joint Transnational Calls for collaborative innovative research projects in Personalised Medicine (PM).
The following are the national funding agencies supporting WODIA:
We thank all of you for your valuable support.
Copyright © Stefan Wagner - WODIA PROJECT